Soil pH Is Like The Cooling System In Your Car!!!
DR. A.J. FOSTER
BLOOMFIELD, MO.
Soil pH in my opinion is similar to the cooling system in a car. The cooling system regulates the operation of the engine, while soil pH regulates and controls many chemical and biological processes that take place in the soil, particularly, plant nutrient availability. Moreover, like the thermostat measures the temperature in your car, soil pH measures the acidity and alkalinity in your soil. pH levels range from 0-14, with 7 being neutral, below 7 acidic and above 7 alkaline. The optimal pH range for most plants is between 5.5 and 7.0, however plants are able to adapt and thrive at pH levels outside this range.
What are the factors that affect soil pH?
Rainfall
Soils formed under low rainfall conditions tend to be alkaline with soil pH around 7 or greater. In contrast, soils formed under high rainfall conditions are more acidic. Thus, most of the soils in southeast Missouri are inherently acidic.
Nitrogen fertilizer
Nitrogen sources contain forms of ammonium that increases soil acidity unless the plant directly absorbs the ammonium ions. The greater the amount of nitrogen fertilizer applied to a soil the greater the potential for soil acidification.
Plants
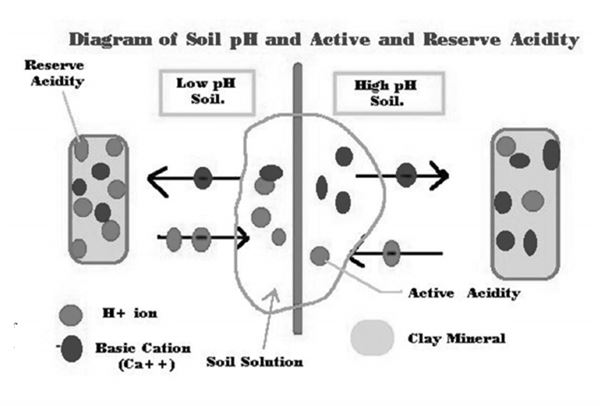
Legumes such as soybeans, alfalfa and clovers are heavy feeders of basic cations such as Ca and Mg. These plants tend to take up more cations in proportion to anions. To maintain the electrochemical balance within their tissues they release H ions from the roots into soil resulting in a net soil acidification. How do I raise the soil pH? Raising soil pH is done by using liming materials containing calcium and/or magnesium, which when dissolved neutralizes soil acidity. The amount of liming material needed to raise the soil pH is dependent on the total amount of H ions held on reserve or on the cation exchange site (Figure 1). Soils testing labs use a buffer solution to determine the amount of exchange acidity needing to be replaced on the exchange site. So, raising the pH is adding liming material to increase the percentage of base cations or base saturation in that soil.
The use of lime to correct soil acidity should be the foundation of any good soil fertility program. CropNutrition.com provides a very detailed overview on soil pH that is a good read for refreshing our basic understanding of soil pH and the importance of lime management in a crop production system. So as you prepare for the 2015 cropping season remember soil pH is a number that deserves respect, the efficiency and the productivity of your entire cropping system depends on it.
The University of Missouri Extension office in each county offers routine soil testing program that provides information on soil pH, Ca, Mg, P, K and OM. For more information on soil sampling and soil testing contact your local Extension office. Remember if you don’t soil test you will be forced to guess!!! ∆
DR. A.J. FOSTER: Agronomy Specialist, University of Missouri